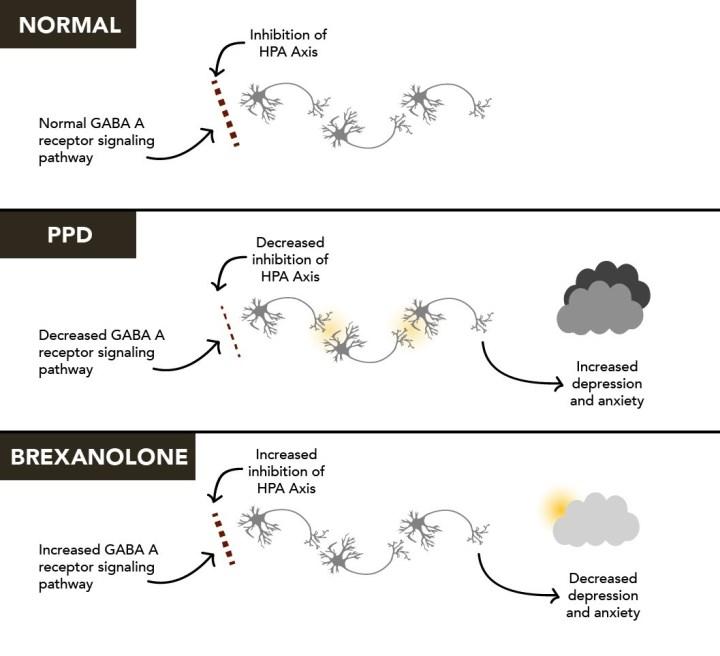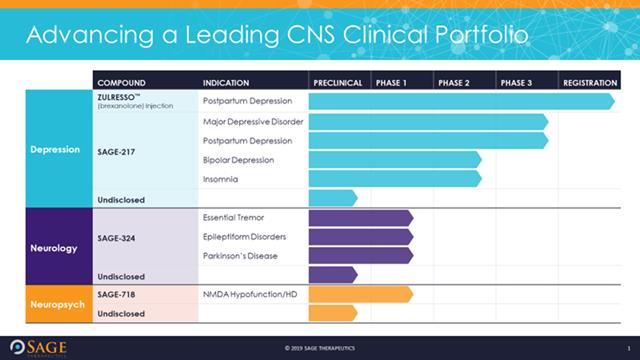
Zuranolone, a breakthrough postpartum depression treatment, was just approved by the FDA. The fast-acting pill can improve symptoms in 3 days
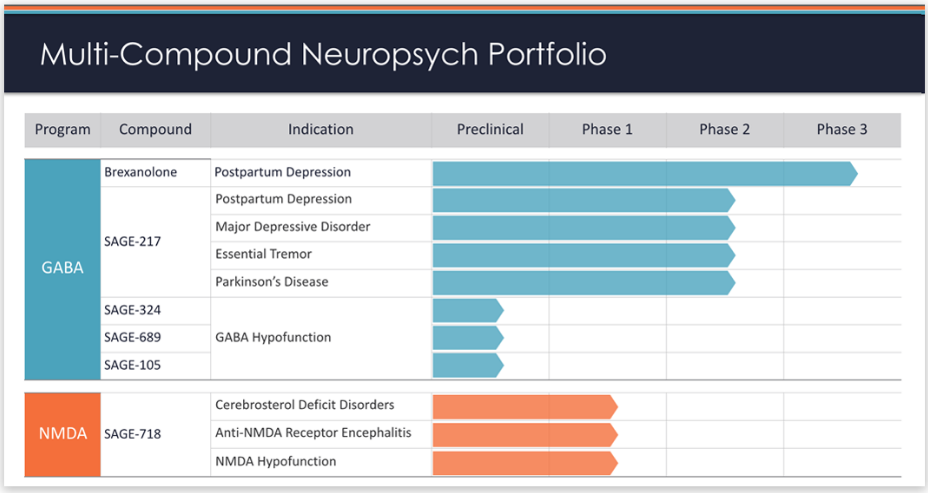
Sage Therapeutics Gets FDA Breakthrough Therapy Designation for Major Depressive Disorder Drug - Equities News

Sage Therapeutics Announces Development Plan for Zuranolone (SAGE-217) Following Breakthrough Therapy Guidance Meeting with the U.S. Food & Drug Administration-CliniExpert

Sage Therapeutics and Biogen Complete Rolling Submission of New Drug Application for Zuranolone in the Treatment of Major Depressive Disorder and Postpartum Depression | Business Wire

Sage Therapeutics Reports Topline Results from Pivotal Phase 3 MOUNTAIN Study of SAGE-217 in Major Depressive Disorder

Zuranolone: Treatment Of Major Depression (MDD) And Postpartum Depression (PDD)! - Industry news - News - Hefei Home Sunshine Pharmaceutical Technology Co., Ltd

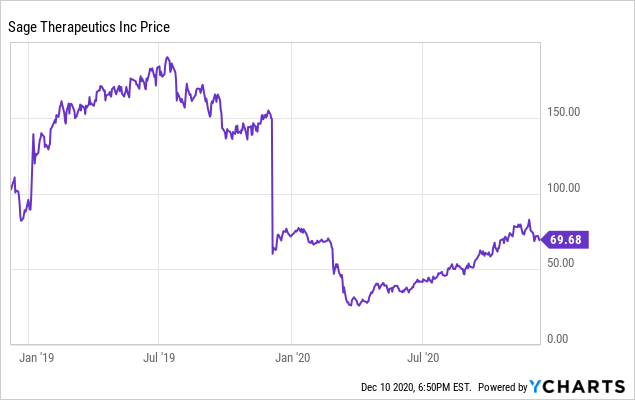
SY Investing on X: "$SAGE (-62% PM) announced pivotal Ph 3 MOUNTAIN results of SAGE-217 in MDD. Study didn't meet primary endpoint. #Fail SAGE-217 showed mean reduction of 12.6 in HAM-D total